Ensuring a safe working environment is paramount in any laboratory, whether it’s for research, diagnostics, or quality control. A crucial tool for achieving and maintaining this safety standard is a well-designed laboratory safety audit checklist template. This isn’t just about ticking boxes; it’s a proactive strategy to identify potential hazards, verify compliance with regulations, and foster a culture where safety is everyone’s priority.
Without regular oversight, even the most diligent lab can inadvertently develop safety gaps. These gaps can lead to severe consequences, ranging from minor accidents and equipment damage to serious injuries, exposure to hazardous materials, or even catastrophic events. Beyond the immediate harm, such incidents can result in significant financial penalties, legal liabilities, and irreparable damage to a lab’s reputation.
That’s why a systematic approach to safety through periodic audits is not merely good practice but an absolute necessity. It allows lab managers and safety officers to stay ahead of potential issues, implement corrective actions swiftly, and continuously improve safety protocols, ensuring the well-being of every individual who steps foot into the lab.
Why a Robust Safety Audit is Non-Negotiable for Your Lab
Implementing a comprehensive safety audit in your laboratory goes far beyond mere compliance; it’s a foundational pillar of operational excellence and ethical responsibility. A thorough audit acts as a diagnostic tool, meticulously examining every facet of your lab’s operations to uncover weaknesses before they escalate into serious problems. It forces a close look at day-to-day practices, equipment integrity, and emergency preparedness, ensuring that theoretical safety policies are effectively translated into practical, on-the-ground actions.
Understanding Regulatory Compliance
Laboratories operate under a complex web of local, national, and international regulations designed to protect workers and the environment. OSHA, EPA, and specific industry standards (like CLIA for clinical labs) set stringent requirements for everything from chemical storage and waste disposal to personal protective equipment and emergency response. A regular safety audit helps ensure your lab consistently meets these legal mandates, avoiding hefty fines, operational shutdowns, and legal battles that can severely impact your institution.
Protecting Your Team and Your Investment
The human cost of an accident in a lab is immeasurable. Injuries, illnesses, or even fatalities not only devastate individuals and their families but also demoralize an entire workforce. A robust audit identifies and mitigates risks that could lead to such tragic outcomes, thereby safeguarding your most valuable asset: your personnel. Furthermore, preventing accidents protects your significant investment in specialized equipment, valuable reagents, and critical research data from damage or loss.
Fostering a Culture of Safety
An audit isn’t just about finding faults; it’s about building a stronger safety culture. When audits are conducted regularly and transparently, they send a clear message that safety is a top priority, not just a secondary concern. This encourages staff at all levels to be more vigilant, report potential hazards proactively, and actively participate in maintaining a safe environment. It transforms safety from a mandate into a shared responsibility, leading to greater awareness and adherence to best practices.
Enhancing Operational Efficiency
Believe it or not, a safe lab is often a more efficient lab. When hazards are minimized, and emergency procedures are clear, there are fewer disruptions, less downtime due to accidents or equipment failures, and a smoother flow of work. Audits can also highlight inefficiencies in workflows that, while seemingly minor, could pose safety risks or impede productivity. Addressing these issues not only makes the lab safer but also more streamlined and productive.
Continuous Improvement and Risk Management
No lab is static, and neither are its risks. New experiments, equipment, chemicals, or personnel constantly introduce new variables. A safety audit provides a structured mechanism for continuous improvement. By regularly reviewing and updating protocols based on audit findings, labs can proactively adapt to changing conditions, manage emerging risks, and ensure their safety framework remains robust and effective over time. This iterative process is crucial for long-term safety success.
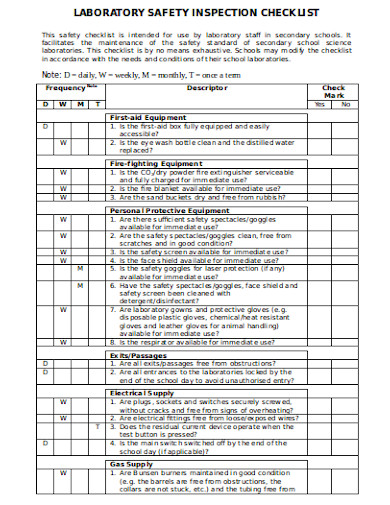
Essential Elements of an Effective Laboratory Safety Audit Checklist
An effective safety audit checklist is more than just a generic template; it’s a living document tailored to the unique risks and operations of your specific laboratory. It should be comprehensive, easy to understand, and designed to prompt thorough inspection and honest assessment. While specific items will vary, there are universal categories that every robust checklist should cover to ensure a holistic evaluation of safety protocols and practices.
To truly provide value, a checklist must guide the auditor through all critical areas, leaving no stone unturned. It should prompt detailed questions about the condition of equipment, the handling of hazardous materials, the readiness of emergency responses, and the general state of the working environment. This detailed approach is what transforms a simple list into a powerful diagnostic tool, helping to identify both glaring deficiencies and subtle vulnerabilities.
Here are some key categories and examples of items typically found in a comprehensive laboratory safety audit checklist template:
- Personal Protective Equipment (PPE) Availability and Usage: Are all necessary PPE items (gloves, safety glasses, lab coats, respirators) readily available, in good condition, and being consistently used?
- Chemical Storage and Handling: Are chemicals properly labeled, stored according to compatibility, and secured? Are Safety Data Sheets (SDS) accessible for all chemicals?
- Hazardous Waste Management: Are waste streams segregated, labeled, and disposed of correctly following regulations?
- Emergency Preparedness: Are emergency eyewash stations and showers functional and unobstructed? Are fire extinguishers current and accessible? Are emergency exits clear? Are staff trained in emergency procedures?
- Equipment Safety: Is all equipment properly maintained, calibrated, and used according to manufacturer instructions? Are safety guards in place?
- Housekeeping and General Lab Environment: Is the lab clean, organized, and free from clutter? Are aisles clear?
- Biosafety Measures: For biological labs, are biosafety cabinets certified, and are proper aseptic techniques followed?
- Electrical Safety: Are electrical cords in good condition, not overloaded, and properly grounded?
Regularly applying a meticulously crafted checklist not only highlights areas for improvement but also reinforces the importance of consistent vigilance. It empowers labs to proactively address potential issues, ensuring that safety protocols are not just written policies but actively practiced principles. This continuous commitment to safety elevates the lab’s operational standards and protects everyone within its walls.
By diligently conducting these audits and acting on their findings, laboratories can cultivate an environment where potential hazards are minimized, and the well-being of staff is always prioritized. It’s an ongoing journey of improvement, where each audit brings the lab closer to an ideal state of operational safety and excellence.